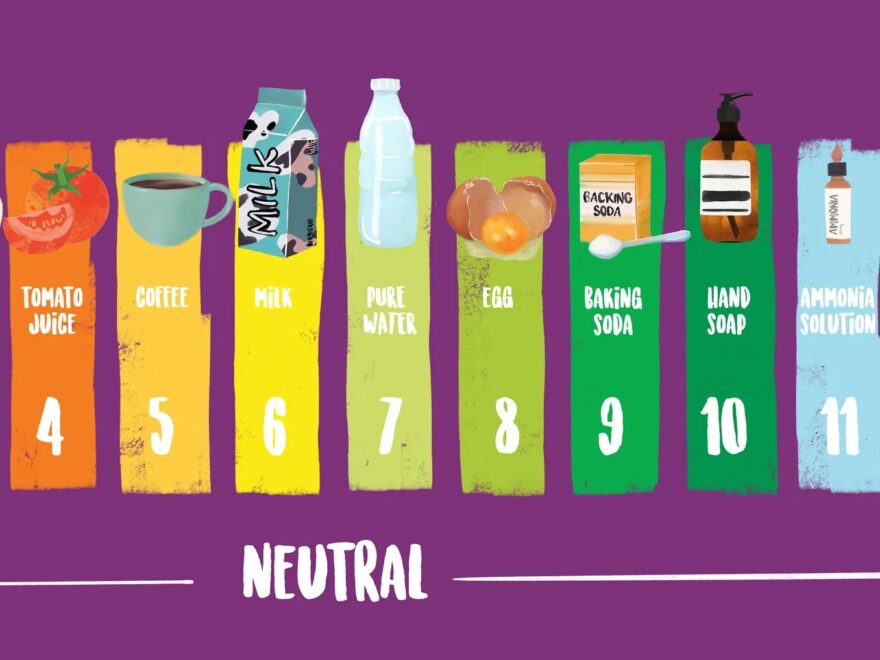
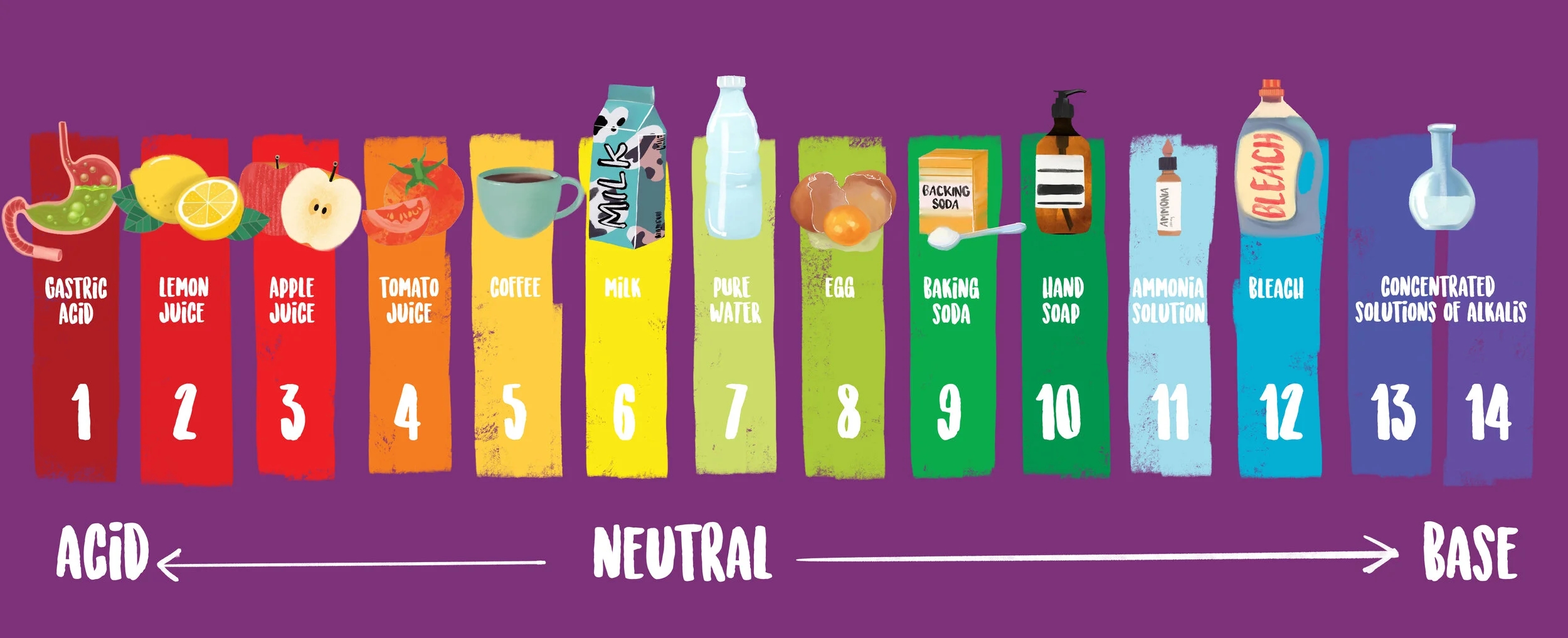
Have you ever wondered how scientists measure the acidity or alkalinity of a substance? Enter the logarithmic pH scale graph, a handy tool used to determine the pH level of a solution. This scale ranges from 0 to 14, with 7 being neutral.
Acidic substances have a pH value below 7, while alkaline substances have a pH value above 7. The further away from 7 a substance’s pH is, the stronger its acidity or alkalinity. For example, battery acid has a pH of 1, while bleach has a pH of around 12.
Logarithmic Ph Scale Graph
Logarithmic pH Scale Graph
The logarithmic nature of the pH scale means that each whole number change represents a tenfold difference in acidity or alkalinity. For instance, a substance with a pH of 4 is ten times more acidic than a substance with a pH of 5.
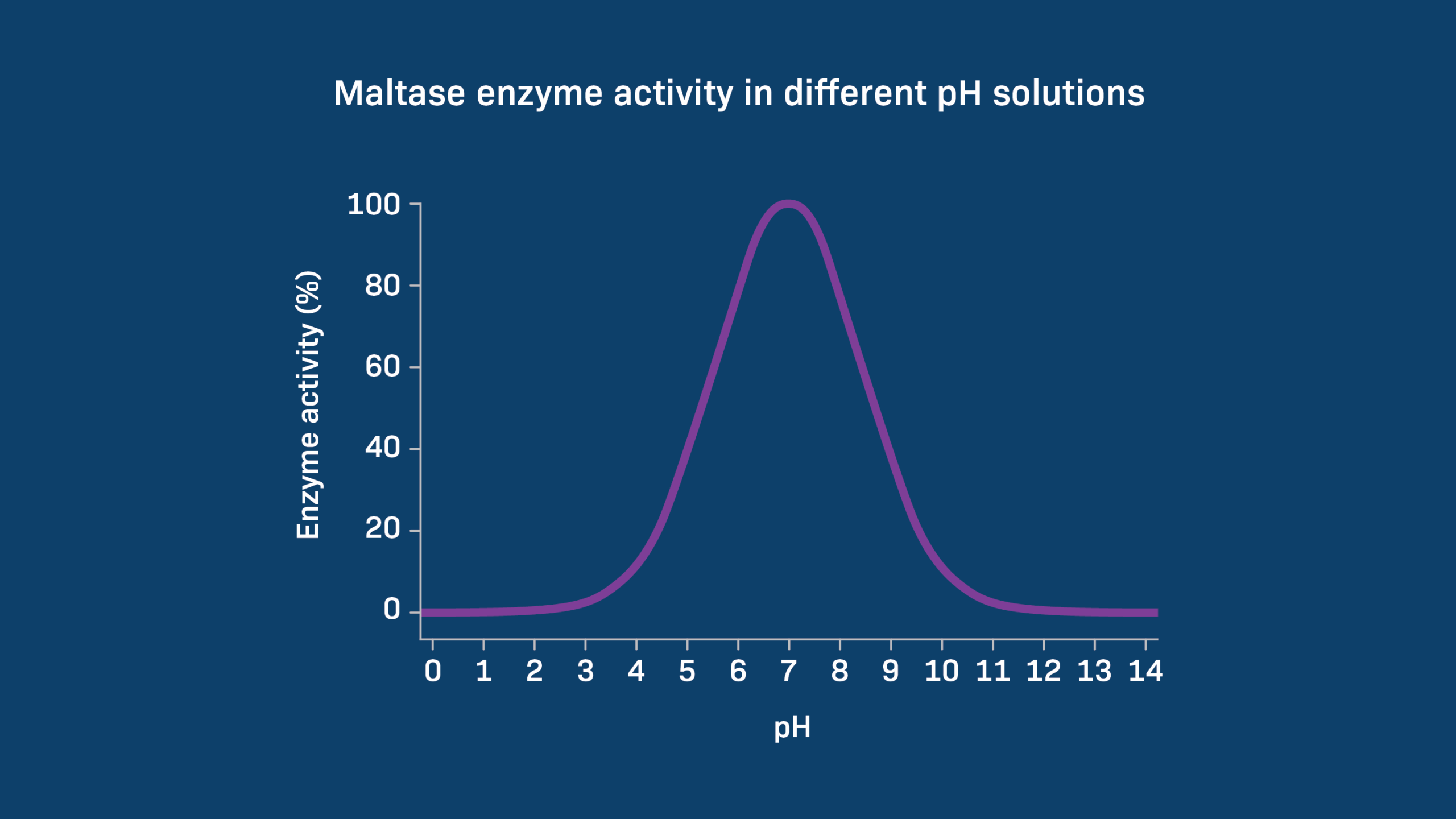
Understanding the pH scale is crucial in various fields, including chemistry, biology, and environmental science. It helps scientists determine the optimal conditions for reactions, study the behavior of enzymes, and monitor water quality in ecosystems.
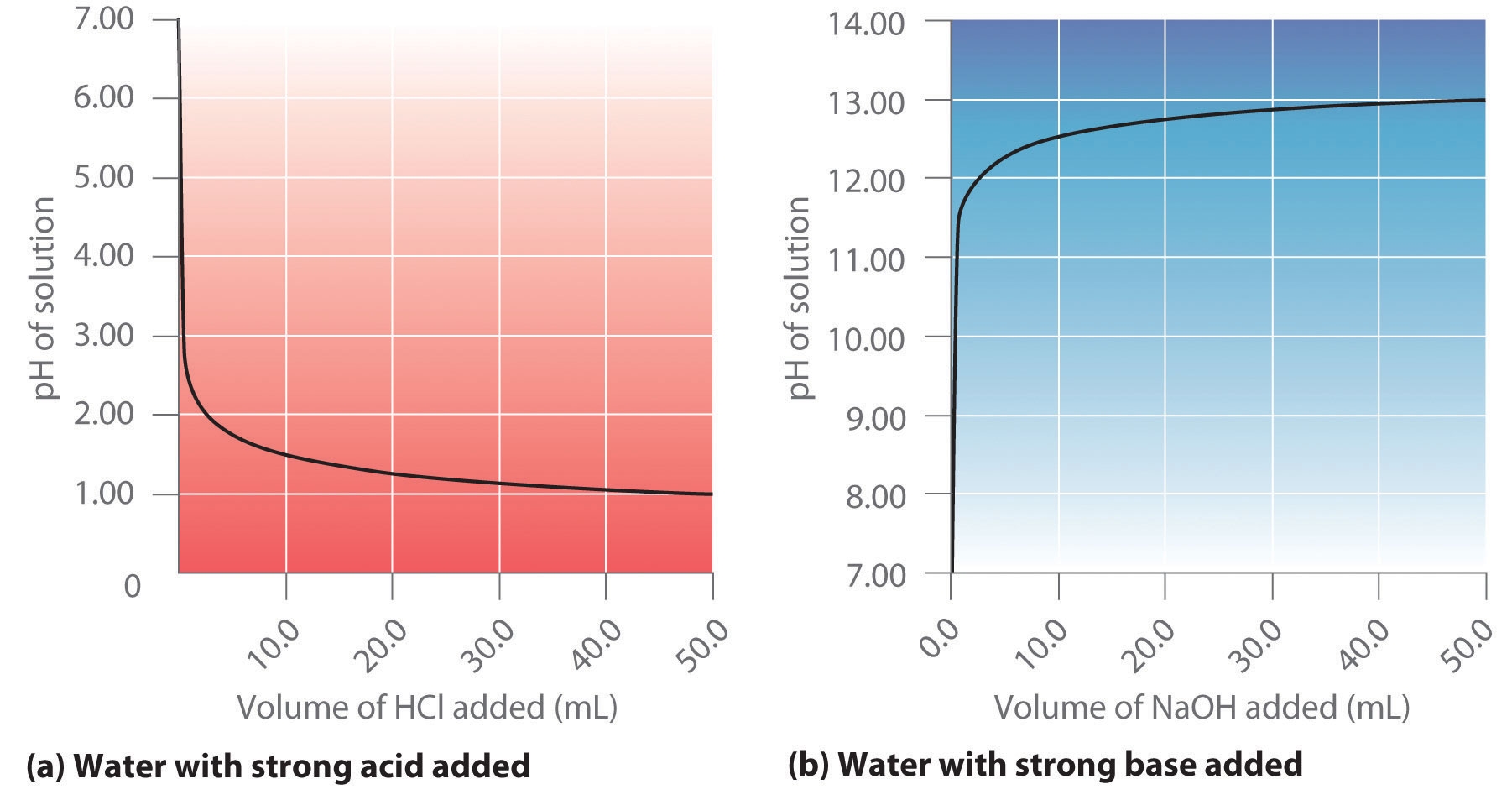
When testing the pH of a solution, scientists use pH indicators or pH meters to obtain an accurate reading. These tools change color or display numerical values based on the concentration of hydrogen ions in the solution, allowing for precise pH measurements.
Whether you’re conducting experiments in a lab or simply curious about the acidity of common household items, the logarithmic pH scale graph is a valuable tool for understanding the chemical properties of substances. Next time you encounter a pH value, remember the logarithmic scale and its implications for acidity and alkalinity.
So, the next time you come across a pH value, you’ll have a better understanding of what it means and why it’s essential in various scientific disciplines. The logarithmic pH scale graph offers a simple yet powerful way to interpret the acidity or alkalinity of substances, making it a valuable tool for researchers and enthusiasts alike.
Acid Base Titrations
How Does A Log Scale Work DataClassroom