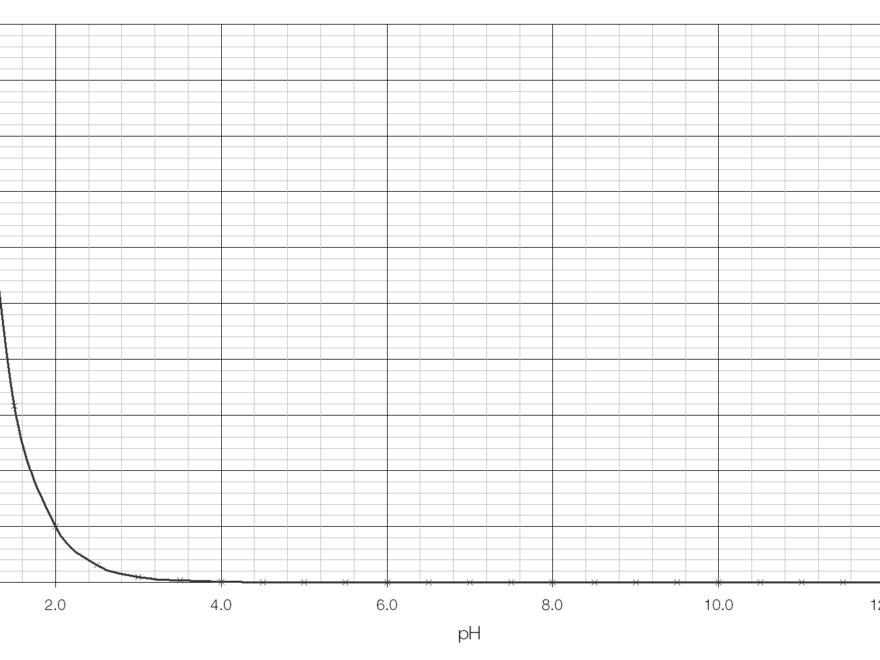
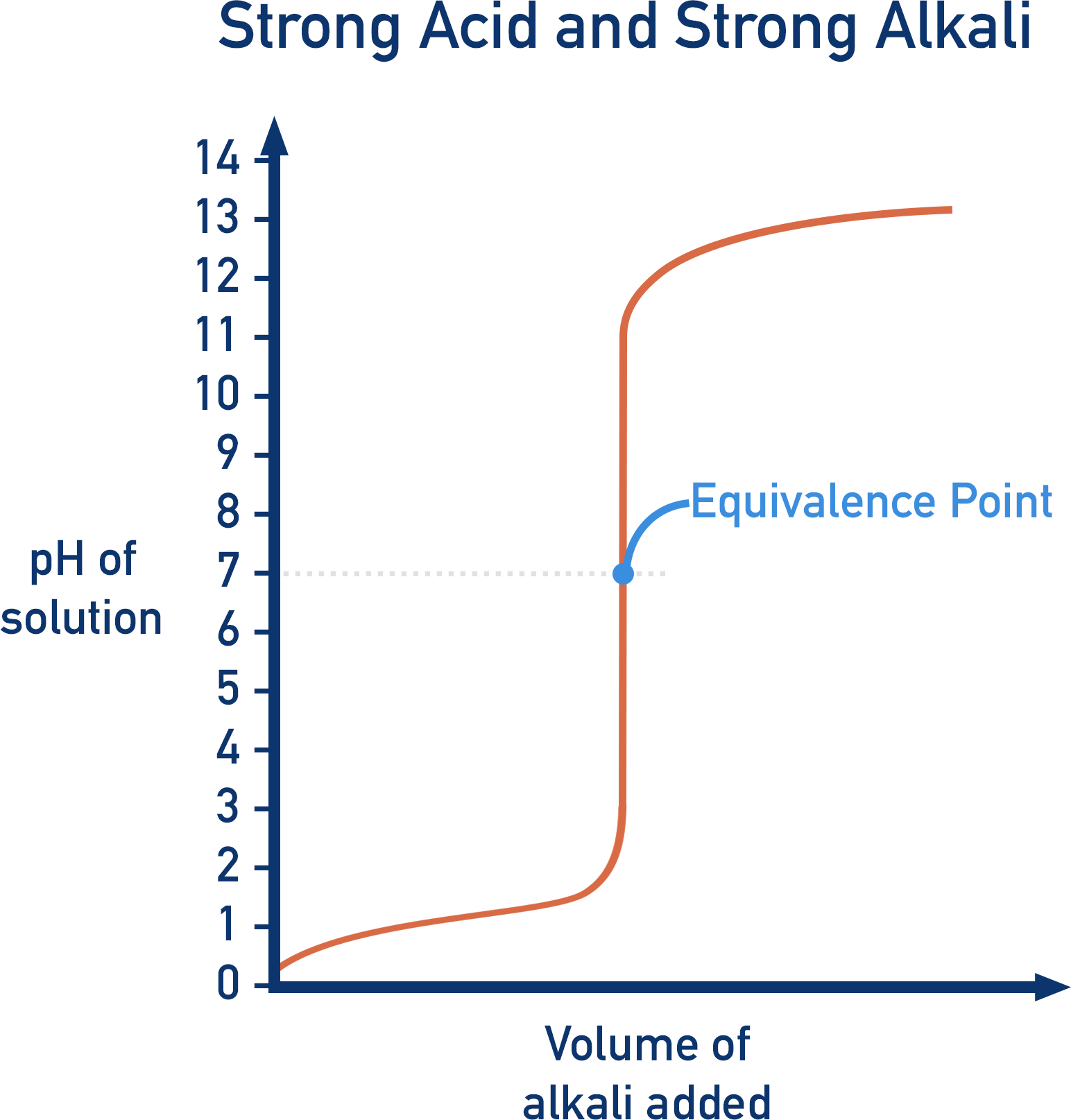
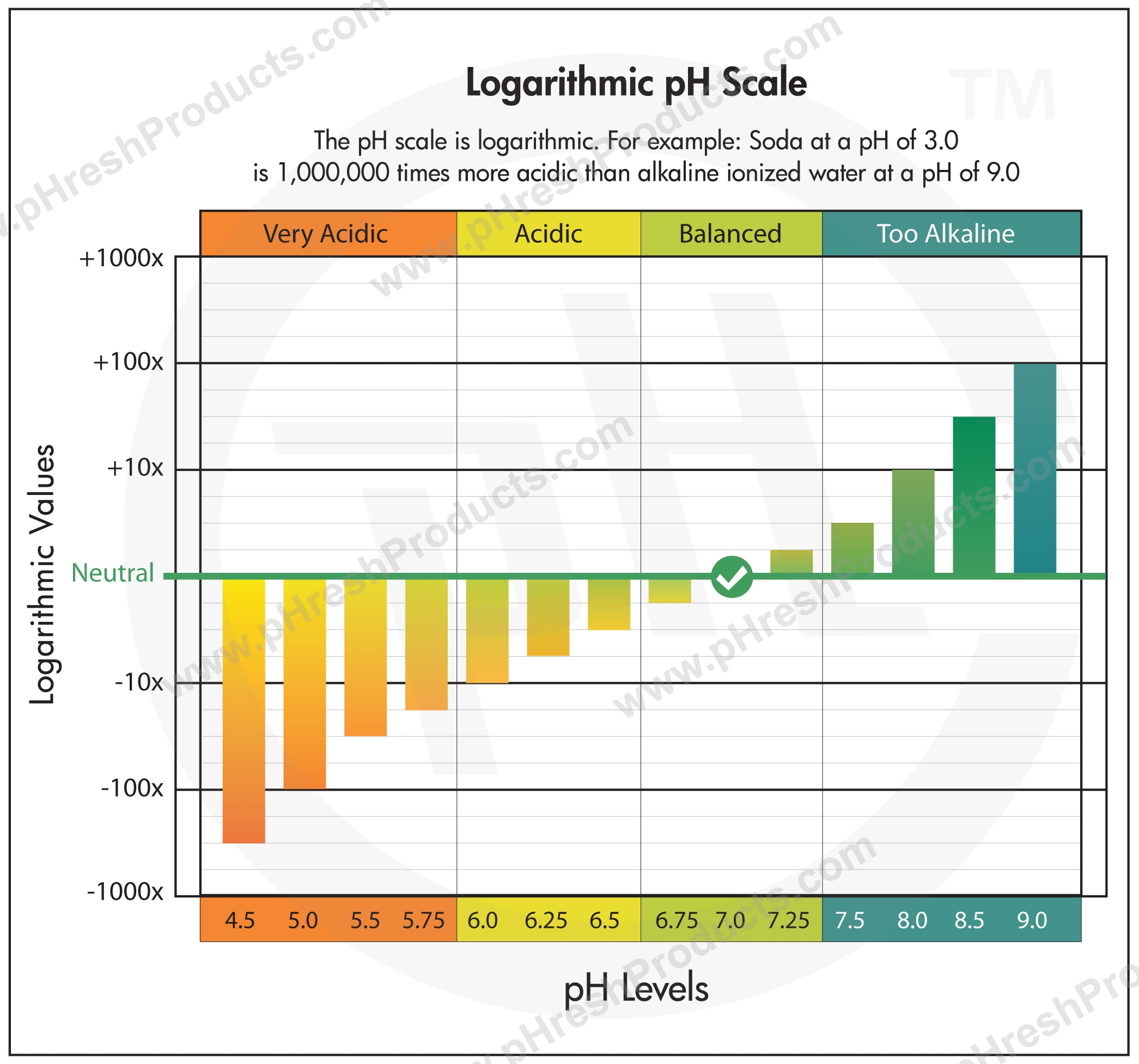
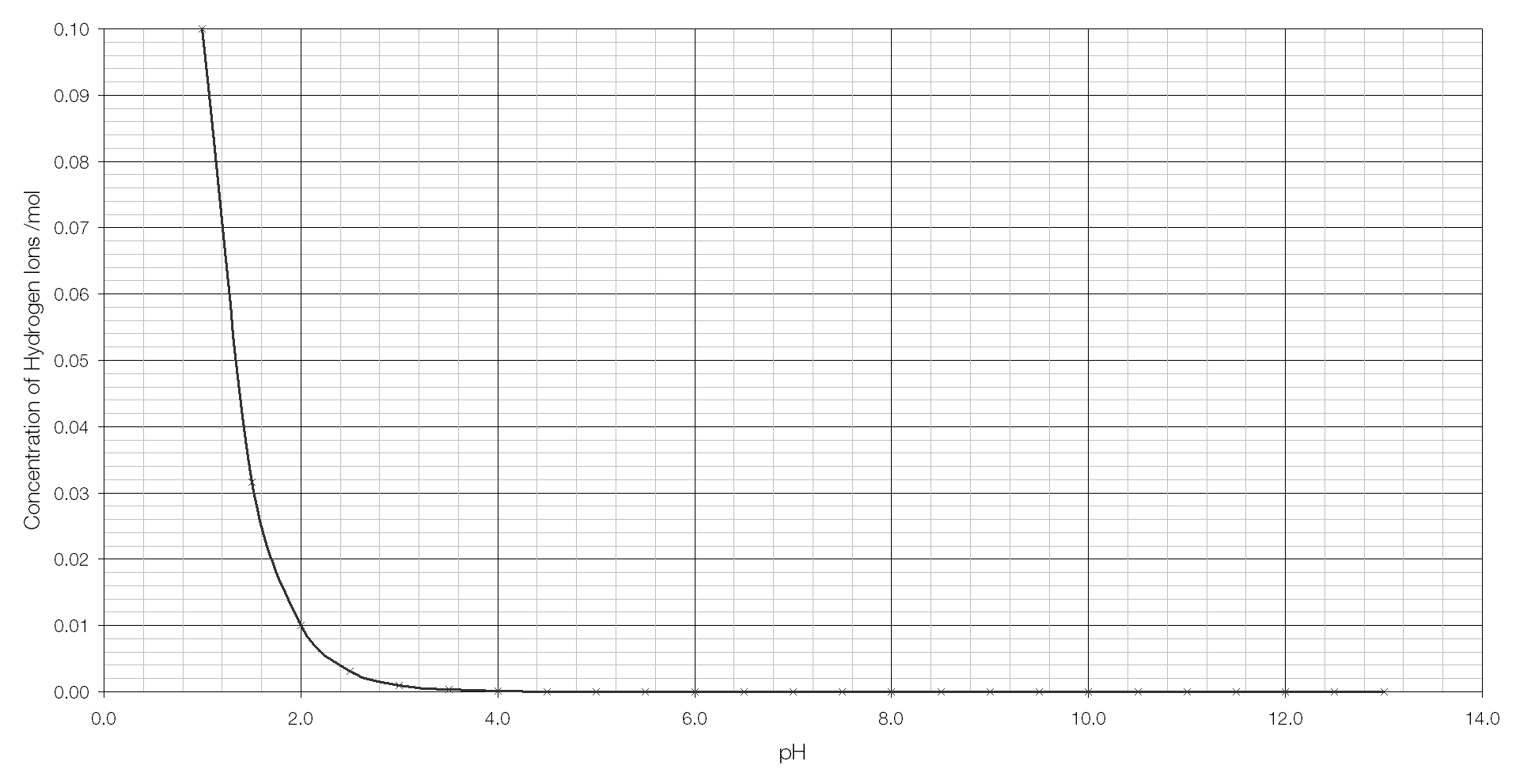
Are you struggling to understand how to read a pH logarithmic scale graph? Don’t worry, we’ve got you covered! pH scales are used to measure the acidity or alkalinity of a substance, with values ranging from 0 to 14.
When looking at a pH logarithmic scale graph, each whole number change represents a tenfold difference in acidity or alkalinity. For example, a substance with a pH of 3 is ten times more acidic than a substance with a pH of 4.
Ph Logarithmic Scale Graph
Understanding pH Logarithmic Scale Graphs
On a pH logarithmic scale graph, as the pH value decreases from 7 to 0, the acidity of the substance increases significantly. Similarly, as the pH value increases from 7 to 14, the alkalinity of the substance increases.
It’s important to note that a pH of 7 is considered neutral, meaning the substance is neither acidic nor alkaline. Substances with a pH below 7 are acidic, while substances with a pH above 7 are alkaline.
When interpreting a pH logarithmic scale graph, pay close attention to the values on the scale and how they correspond to the acidity or alkalinity of the substances being measured. Understanding this relationship is key to interpreting the data accurately.
Next time you come across a pH logarithmic scale graph, remember that each whole number change represents a tenfold difference in acidity or alkalinity. With this knowledge, you’ll be able to decipher the graph with ease and understand the relative acidity or alkalinity of the substances being analyzed.
Now that you have a better understanding of how to read a pH logarithmic scale graph, you’ll be better equipped to interpret data and draw meaningful conclusions. Remember, practice makes perfect, so don’t hesitate to dive into more graphs to sharpen your skills!
Logarithmic PH Scale PHresh Products
Logarithmic Scales MrReid